Solvents for HPLC
For modern HPLC, high purity solvents are increasingly needed as mobile phases. In general, users choose between three different classes of purity for their individual analysis: HPLC grade, Gradient grade and LC-MS grade.
But what's the difference between the different qualities in regards to chromatography and other high purity solvents? And why is it important to choose a solvent with reference to the performed application?
All qualities have the same basic material and a nominal high purity with low traces of xenobiotics (99.8 - 99.9 %, depending on the product) in common. However, it is precisely these minimal residues that can cause problems in HPLC, depending on the detection technique, so in this case "pure" is not the same as "pure".
In the following, we will look at the differences of the three levels of solvent purity and when to choose which quality for your application.
HPLC grade solvents
HPLC grade describes a solutions quality, that is mainly designed for isocratic separations applying UV and fluorescence detection. These solvents are especially characterised by high purity combined with high UV permeability at all relevant wave lengths, low inherent fluorescence and waste steam pressure as well as low acidity and alkalinity.
The solvents undergo special treatment stages to remove potentially interfering substances. This helps minimising spurious peaks and allows for low ambient noise.
After the treatment these solutions are tested specifically for aptitude for fluorescence detection in HPLC. Through this quality control continuous purity of solvents as well as reproducibility for your measurements can be guaranteed.
Gradient grade solvents
For chromatographic separation in HPLC, gradient elutions are often used in addition to isocratic elutions. In this case the composition of the mobile phase doesn't change during the measurement, so that e.g. in reversed-phase chromatography the organic fraction increases.
The use of solvents not specifically designed for gradient separation can cause a significant increase in the baseline for UV detection and may affect the sensitivity of the method.
The following figure shows a gradient profile to demonstrate the extraordinary results that can be attained with Gradient grade acetonitrile. Only a low drift of the base line can be recorded.

Fig. 1: Drift of the base line using Gradient grade as solvent.
LC-MS grade solvents
With the success of (ultra) high pressure liquid chromatography-mass spectrometry (UHPLC-MS), many new demands were placed on the solvent used to achieve high detection sensitivity.
The focus of using mass spectroscopy is on high ionisation efficiency with low ion background. Therefore, the solvents used are purified; removing trace elements, organics and (metal) ions is the first priority as these can lead to an increased ambient noise and adduct formation with the substances to be analysed. The result is a decreased detection sensitivity.
For quality control, all solvents are tested against reserpine (m/z 609.49) as standard. The following figure shows the scan of a positive and negative ESI and APCi mode. The entire mass range tested shows a low noise environment and no signals from contaminants.
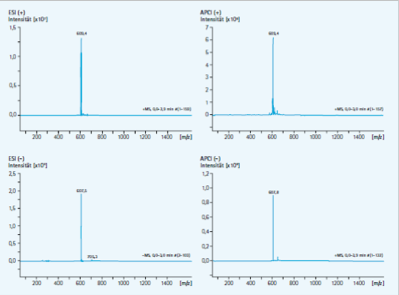
Fig. 2: Noise environment of LC-MS measurements with LC-MS grade solvents.