costs from $250
products
Selection of a suitable HPLC column
Since the beginning of high-performance liquid chromatography (HPLC) in the 1960s, the method has been steadily developed and refined. This applies equally to the materials used. There are now ten thousand different HPLC columns from many different manufacturers – solely in our HPLC Column configurator you can find more than 60.000 different columns. This selection naturally offers great advantages - among other things there are very specific separation columns for almost every separation problem - at the same time, the user is spoiled for choice. The following text provides guidance and helps in the selection of the appropriate HPLC column.
Column Hardware
Most HPLC columns consist of 316 steel. The advantage is that the steel is pressure-resistant and also relatively inert to corrosion. For a good separation performance it is important that the inside of a column has no roughness, scoring or microporous structures (Meyer 2009, p. 110).
Base Material
Silica Gel
A popular base material in HPLC systems is silica gel; it consists of Si atoms bridged by oxygen atoms (Meyer 2009, p. 119). A disadvantage of silica gel is that a high pH leads to the dissolution of the silica skeleton. Silica gel columns can be used in the pH range from pH 1 to 8 (Meyer 2009, p. 120). A low metal content makes it possible to increase the chemical stability of highly pure silica gels. On the surface, silica gel carries OH groups (silanol groups). These silanol groups can be chemically modified so that stationary phases with specific properties are obtained (Meyer 2009, p. 121). In addition to its mechanical stability, silica gel has the advantage of a low-cost and simple synthesis. Likewise, the surface modification is simple, low-priced and flexible. The pore size can be produced with a narrow distribution of silica gel (Kromidas 2014, p. 246).
Polymer Columns
HPLC columns based on polymer are also available for RP-HPLC (reversed phase HPLC). Usually polystyrene-divinylbenzene columns are used, which offer a higher pH stability (pH 1-13) against the silica gel columns. For strongly acidic or basic eluents they represent an interesting alternative (HPLC column 2016). Especially for gel permeation chromatography, styrene-divinylbenzene is an important stationary phase (Meyer 2009, p. 126).
Shape of the column material
Fully Porous Particles
In addition to the traditional full-porosity phases, there are now also coreshell phases, monolithic phases and phases for UHPLC with small particle sizes.
Most of the particles used in HPLC are the full-porosity particles. Conventional grain sizes are 3, 5 or sometimes also 10 μm. When the particles are full-bodied, the entire inner structure is porous and can be compared, for example, with a sponge (Meyer 2009, p. 116).
Coreshell Particles
Coreshell particles have a solid core and only the outer shell is porous. This solid core results in increased separation performance. The particles have a more uniform particle size distribution and can therefore be packaged better, the A-term of the Van-Deemter equation (Figure 1, p. 5) is decreased. In addition, the mass transfer of the molecules into the pores (C-term of the Van-Deemter equation) is accelerated. A disadvantage of the coreshell particles is the lower loadability against fully porous materials.
Monolithic Phase
Monolithic columns consist of a single piece of porous material, for example silica gel or organic polymer. Thus, the chromatographic bed does not consist of individual particles but of a porous rod. Monoliths have a separation capacity that is comparable to 3 μm packed particle columns (Meyer 2009, p. 118). A drawback is that, for monolithic phases, the availability of selectivities is still limited compared to totally porous materials (Kromidas 2014, p. 152).
Bound Phase
The variety of available stationary phases (reversed phase, normal phase, phenyl phases etc.) of various manufacturers contributes to the fact that it is not always easy to quickly find the right HPLC column.
A common reversed phase C18 column can be used for many analyzes. In order to decide which stationary phase is suitable, the molecule to be investigated should be considered in the first step. If one wants to analyze a very polar molecule, there is a possibility that it does not interact with the C18 material and thus elutes in the dead time. In this case, for example, C4, CN, diol or phenyl phases are suitable. If on the other hand the analyte is too non-polar it can irreversibly adhere to the material and thus no longer elute from the column. To avoid this you can try a silica, CN or NH2 phase. This usually happens when the analyte is soluble only in heptane or hexane (Kromidas 1997, p. 32-33).
Once the decision is made for the phase, the first hurdle is taken. However, it becomes even more complicated since one has to decide between different dimensions and specifications for the column to be used, such as particle size, pore size, internal diameter, diameter and carbon content.
Table 1: Overview of the bound phases (HPLC column 2016)
| Phase Name | Formula | Structure | Application | USP Number | Possible Materials |
|---|---|---|---|---|---|
| Octylsilane (C8), not endcapped | -(CH2)7-CH3 | 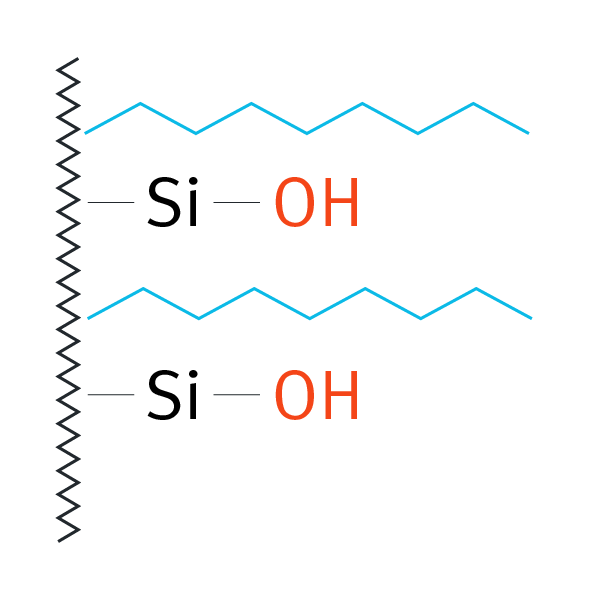 |
medium polar compounds | L7 | Waters Symmetry C8 |
| Octylsilane (C8), endcapped | -(CH2)7-CH3 | 
| medium polar compounds | L7 | Merck Chromolith Performance RP-8 endcapped |
| Octadecylsilane (ODS, C18), endcapped | -(CH2)17-CH | 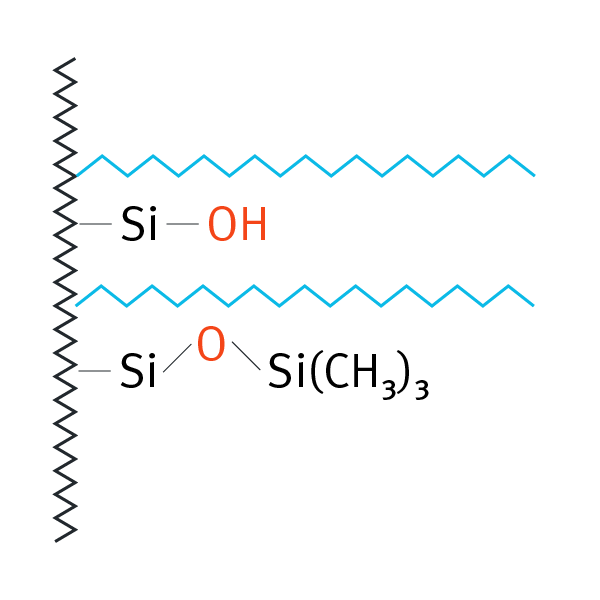 |
non polar and medium polar compounds | L1 | Altmann Reprosil Pur C18-AQ |
| Phenyl | -(CH2)6-C6H5 |  |
medium polar, unsaturated compounds | L11 | Altmann Reprosil 100 Phenyl |
| Phenyl Hexyl | -(CH2)6-C6H5 |  |
medium polar, unsaturated compounds | L11 | Waters XSelect Phenyl Hexyl |
| Pentafluorophenyl (PFP) | -(CH2)3-C6F5 |  |
unsaturated, halogenated, polar compounds | L43 | Altmann Reprosil Fluosil PFP |
Particle Size
Smaller particle sizes yield better resolution than larger particles. Larger particles on the other hand produce a lower back pressure in the HPLC system. A uniform particle size distribution is particularly important in addition to the size of the particle. An increased proportion of small particles increases the backpressure; if too many larger particles are contained, the material loses separation performance due to a lower ground count. If a wide particle size distribution is used, the material can also be poorer (Kromidas 2014, p. 290). The size of the particle has an influence on the quality of the resolution since the size of the particles affects the A and C terms of the Van Deemter equation.
Figure 1: Terms of the VanDeemter equation (chemgepedia 2016)

Optimal separation performance is achieved with as small a floor as possible, thereby increasing the total number of separating floors in the column. Especially when the A and C term can be minimized, it is possible to achieve a good separation performance over a large flow range.
Pore Size
The pore size to be used depends on the size of the molecule to be analyzed: the larger the molecule, the larger the pores should be. Pore sizes are usually given in angstroms, corresponding to 10 Å = 1 nm. For small molecules, a column up to 120 Å can be used. For large biomolecules, for example, columns with pore sizes of 3000 Å are suitable (Kromidas 2014, p. 291). For biomolecules the selection of the correct pore size is particularly important as it is available in a wide range of sizes. The smaller the pore, the more surface and interaction possibilities the phase has. This allows them to be loaded higher (Kromidas 2014, p. 291).
Table 2: Column selection tool biomolecules (YMC 2015)
| MW | Å | C18 | C8 | C4 |
|---|---|---|---|---|
| - | 120 | +++ | ++ | + |
| >5000 | 200 | ++ | +++ | ++ |
| >20000 | 300 | + | ++ | +++ |
(+++) = very good; (++) = good; (+) = mediocre
Inner Diameter/Length
Depending on the type of analysis and amount of the substance to be analyzed, the dimension of the HPLC column should be selected. The HPLC system also limits the variety of available dimensions (Moldoveanu & David 2013, page 227).
Table 3: Classifications of HPLC columns by dimensions (Moldoveanu & David 2013)
| Inner Diameter (mm) | Length (mm) | Flow Rate (mL/min) | Sample Quantity (µg) | |
|---|---|---|---|---|
| Preparative | >25 | 300 or larger | >20 | >25,000 |
| Semi-Preparative | 10 | 250 or larger | 5-10 | 10,000-20,000 |
| Analytically Conventional | 3 | 4 | 4.6 | 50 | 100 | 150 | 250 | 0.5-2 | 50-200 |
| Analytically Narrowbore | 2 | 2.1 | 50 | 100 | 150 | 250 | 0.2-0.5 | 20-100 |
Conclusion
It is now clear that when choosing a suitable HPLC column many different parameters have to be considered. There is usually not the one right column, but you often have a variety of options that might be appropriate for the analysis. If the column parameters are selected specifically for the analytes and the instrumental equipment is taken into account, it can be assumed that a suitable chromatographic separation is obtained.
If you are still unsure about the column selection, our product specialists from Analytics Shop will be happy to advise you. We will be pleased to provide you with suggestions without any obligation from various manufacturers depending on the separation problem. Sometimes there is the possibility to get a free test column, just contact us.